Neural Control of Micturition in a Rat Model of Parkinson's Disease
Natalie Poonam Phagu
Faculty Mentor:
Dr. William F. Collins, III
Stony Brook University
Key Words: basal ganglia, bladder pressure (BP), dopamine (DA), external urethral sphincter (EUS), inter-contraction interval (ICI), lower urinary tract (LUT), micturition reflex, periaqueductal grey (PAG), substantia nigra pars compacta (SN).
Abstract
Parkinson’s Disease (PD) is a neural disorder characterized by atypical motor function as well as dysfunction of the lower urinary tract (LUT). Micturition (urine evacuation/voiding) and continence (urine storage) are modulated by the neurotransmitter dopamine (DA) through innervation in the Basal Ganglia region of the midbrain, including the substantia nigra pars compacta (SN). Loss of these dopaminergic pathways and the resultant loss of D1 receptor-mediated inhibition have been linked to the development of LUT dysfunction in PD (Araki et al, 2000 and Ogawa et al, 2006). Thus, through lesioning of the SN via 6-OHDA, this study has created a commonly used rat model of PD for the purpose of improving upon cystometric study and evaluation of bladder dysfunction in PD.
Animals were studied at one week, two weeks, and four weeks post-lesion, whereas the most common study of this kind is carried out over a period of two weeks. Previous studies have indicated that unilateral lesion of the nigrostriatal pathway leads to bladder hyperactivity two weeks post-lesion (Yoshimura et al, 2003). The goal of the present study is to evaluate the full time course of data following 6-OHDA lesion-induced changes in LUT function, including activity of the bladder and external urethral sphincter (EUS). Preliminary results support the hypothesis that a 6-OHDA lesion in the SN produces a transient increase in voiding frequency within the first two weeks, with recovery of urinary function by four weeks post-lesion. Meanwhile, motor deficits (e.g. contralateral rotation) develop within a few days and persist for at least four weeks. These results suggest that following loss of dopaminergic neurons, compensatory mechanisms are activated that serve to restore LUT function. Future studies will address this hypothesis. Identification of compensatory mechanisms, particularly ones involving plasticity of remaining dopaminergic neurons, would facilitate development of new treatment options for PD patients.
Introduction
The micturition reflex involves coordination between the smooth and striated muscle of the bladder, urethra, and urethral sphincter in order to store and eliminate urine. While it can occur involuntarily, in adult humans it is under voluntary control due to learned behaviors (de Groat et al, 2013). Peripherally, micturition is modulated by the parasympathetic (Acetylcholine (Ach) and Nitric Oxide (NO)), sympathetic (Norepinephrine (NE)), and somatic nervous systems (Ach) (de Groat et al, 2013). Urine is expelled from the bladder when acetylcholine excites the smooth muscle of the bladder and nitric oxide inhibits the smooth muscle of the urethra via parasympathetic pathways. Continence, on the other hand, is controlled by sympathetic pathways that inhibit detrusor activity and activate the muscles at the base of the bladder and the urethra in order to prevent the leakage of fluid ((Fowler et al, 2008). Numerous neurotransmitter molecules have also been implicated in the control of the bladder and external urethral sphincter (EUS) muscle by the central nervous system (CNS), including glutamate, γ-aminobutyric acid (GABA), NE, 5-hydroxytryptophan/serotonin (5-HT), and dopamine (DA). This study modifies input to the micturition reflex acting centrally, with a focus on selective loss of DA via 6-hydroxydopamine (6-OHDA) lesion of the substantia nigra pars compacta (SN) to create a rat model of Parkinson’s disease (PD).
In prior studies, 6-OHDA lesioned rats have exhibited increases in peak bladder pressure during voiding and decreases in inter-contraction interval. These effects mimic blockage of D1 receptors, leading to the conclusion that the micturition reflex is controlled primarily by dopaminergic input through D1 receptors in which there are normally increased levels of dopamine and glutamate and decreased levels of GABA (Kitta et al, 2008). It is suspected, therefore, that reduced inhibition caused by diminished dopamine levels contributes to bladder hyperactivity. Tracing methods involving use of pseudorabies virus (PVR) have indicated the activation of the periaqueductal gray (PAG) during micturition, and single unit recordings have been used to confirm the predominantly inhibitory effect of dopaminergic neurons in the SN and ventral tegmental area (VTA). However, stimulation of the PAG depends on the state of the bladder and also the exact location of the PAG, causing excitation when the bladder is not filled to capacity, and inhibition when filled and active (de Groat et al, 2013). While some agree that the PAG is absolutely essential to the micturition control pathways, others argue that it is only a relay and coordination station for transmitting information about the filled state of the bladder to higher brain centers (de Groat et al, 2013). In this study, we rely on the hypothesis that in anesthetized animals, the PAG provides input to the pontine micturition center (PMC) in order to control micturition reflexively, acknowledging that the awake rat might not void purely reflexively.
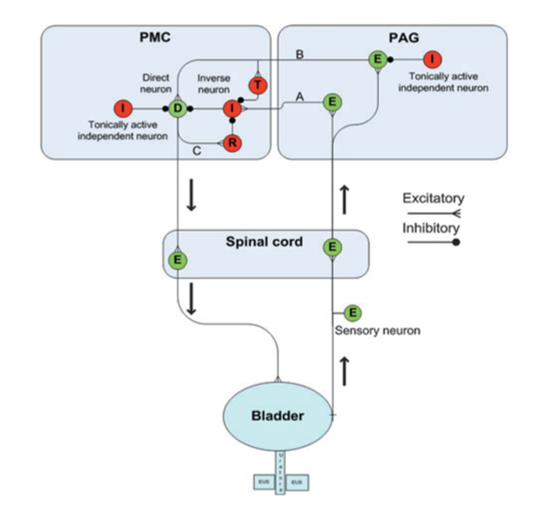
Figure 1: Diagram depicting neural control of bladder storage and voiding by the pontine micturition center (PMC) and periaqueductal gray (PAG) regions of the brain. Excitatory neurons are colored green and inhibitory are red. An excitatory neuron (E) in the PAG incites an inverse neuron (I) in the PMC via pathway A to inhibit the direct neuron (D) so that the bladder is not activated and continence is maintained. Another excitatory neuron (E) in the PAG activates both the direct neuron (D) and a transiently active neuron (T) in the PMC via pathway B, but these pathways are inhibited by independent neurons (I) in the PMC, which serve to promote continence. When firing of the pathway on the right reaches a critical level, excitation overcomes inhibition, and pathway C is also activated such that a reciprocal neuron (R) inhibits the inverse neuron (I). Together, these allow for a strong bladder contraction and subsequent emptying of the bladder (Reproduced from de Groat et al, 2013).
In this study, the SN has been chosen as the area of lesion since dopaminergic projections begin there and terminate in the neostriatum (caudate-putamen) (Moore et al, 1978). However, as pointed out by Sakakibara et al (2012), it is important to note that Parkinson’s disease involves the degradation of multiple locations and systems of the brain other than those involving dopamine, and there may be variation in the symptoms presented by either sex. For example, males have a greater number of alpha-receptors in the urethra. In this study we are specifically targeting the SN in order to inhibit dopaminergic control of micturition, with little control over the condition of various connected systems. Although the PAG is believed to be the region where the switch occurs between storage and voiding, there is no clear understanding of the connection between the SN and PAG. The working hypothesis considers that D1-receptor activation is excitatory. However, the fact that D1 receptor activation leads to inhibition of micturition suggests that there is activation of GABA, which leads to indirect deactivation of the micturition reflex. The SN primarily projects to the Caudate but electrical stimulation to the SN causes inhibition of micturition and administration of dopamine systemically also inhibits bladder contraction (Yoshimura et al, 1992). Dopaminergic neurons from the A11 catecholamine cell group project throughout the spinal cord and regulate sympathetic activity (Skagerberg et al, 1985 and Takada et al, 1988). The relationship between the SN and PAG, along with the role of the VTA, is a subject for further study. The SN is of interest due to the implications that loss of dopaminergic neurons in the region to which it projects (i.e. the striatum) have for motor dysfunction in PD (Ogawa et al, 2006) and their synchronous development with LUT symptoms (Sakakibara et al, 2012). The purpose in carrying out this study is to confirm the findings of previous studies via establishing a similar model, while going on further to quantify EUS measurements.
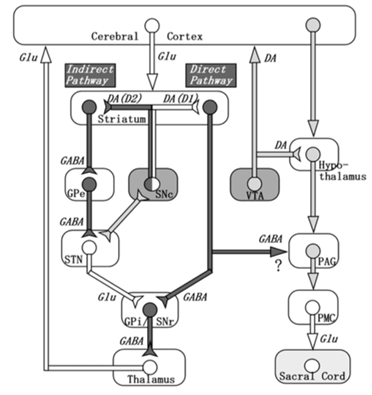
Figure 2: Depiction of connections between the basal ganglia (left) and the known micturition centers of the brain (right). Dark-colored pathways are inhibitory and white-colored pathways are excitatory. Micturition is modulated by nigrostriatal dopamine via D1 receptors (net effect is inhibition of micturition) and D2 receptors (excitatory effect on micturition), and by GABA (inhibitory). Action potentials in the SN cause the release of dopamine in the striatum, leading to activation of the D1 (direct) pathway. This inhibits the output nuclei of the basal ganglia and may lead to GABAergic inhibition of the micturition pathway on the right. Note that the effect of the nigrostriatal DA system on the PAG is indirect and there are also other dopaminergic projections coming from the hypothalamus and influencing PAG activity and in turn, micturition. (Reproduced from Sakakibara et al, 2012).
As many as 55 percent to 80 percent of PD patients may be affected by LUT dysfunction, especially detrusor overactivity, but their symptoms are resilient to and sometimes worsened by traditional treatments for motor dysfunction in PD patients (Kitta et al, 2006 and Sakakibara et al, 2012). There is some debate as to whether urge incontinence and other symptoms commonly attributed to PD are truly associated with the disease itself or merely a consequence of the biological aging process of mammals. However, young adult animals were used in these experiments and not subject to such concerns. Another concern is that patients suffering from PD may take medications that influence autonomic pathways, leaving symptoms underreported and a consequent underrepresentation of the true magnitude of LUT dysfunction in PD. Therefore, consensus upon the association between bladder anomalies and PD is vital to the development of therapeutic interventions to treat LUT dysfunction in addition to traditional PD medications. One such promising example is an A2A (adenosine) receptor antagonist, which suppresses bladder overactivity in rats with PD (Kitta et al, 2012).
While much attention has been paid to bladder hyperactivity in numerous studies including those by de Groat, Yoshimura, and Kitta, there has been very little focus on the role of EUS activity in LUT symptoms such as urinary frequency in PD and the exact mechanisms behind bladder and EUS synchronization. The EUS plays an essential role in the coordination of this reflex. Because changing patterns in EUS activity may contribute to LUT dysfunction, it is essential that the present study quantify the change in EUS activation following a 6-OHDA lesion. In limited efforts where EUS activity has been studied under the influence of D2 receptor agonists, it was determined that while there is dopaminergic input to the EUS motoneurons, this influence is of minimal consequence. D2 agonists produced minor hyperactivity of the urethra as well as delayed or only partial relaxation of the sphincter muscle following voiding (Ogawa et al, 2006). In addition, the majority of PD patients with LUT dysfunction have symptoms characterized by difficulties in storage (e.g. nocturia) as opposed to voiding disorders (Sakakibara et al, 2012). This indicates that underlying dysfunction is most likely bladder-related. However, this does not rule out possible changes in EUS function that may contribute to the LUT dysfunction observed in PD.
This study also emphasizes the benefit of including EUS measurements in order to gauge the level of activation of the system following surgical procedures in acute cystometry. This is to ensure that the EUS has had adequate time to return to rest such that false positive data displaying hyperactivity is excluded from data collection. If it can be established that PD is indeed concomitant with LUT dysfunction in humans, this may allow for early screening and detection of the disease. The data collected in this study include EUS parameters such as the duration of EUS bursts, frequency of bursting, EUS EMG area, and sustained activity of the muscle following voiding (i.e. gradual decrease in muscle activity before returning to rest). High frequency of EUS bursting may indicate more efficient emptying of the bladder or perhaps increased excitability. The study also maintains animals at various lengths of time and pays close attention to the progression of data over time instead of only over a short period. We have also included information about voiding frequency and volume collected by placing the rat in a diuresis chamber. Thus, the present study aims to examine the observed transient effect of 6-OHDA on bladder dysfunction in rats, which may lead to further analyses of the mechanism through which normal bladder function is restored.
Materials and Methods
Animal Preparation
Experiments were performed using female Sprague Dawley rats (240-300g) in accordance with guidelines provided by the Institutional Animal Care and Use Committee at Stony Brook University. 6-Hydroxydopamine (6-OHDA) (2 ml, 0.48 mg/ml in 0.9% NaCl, 0.1% ascorbic acid vehicle) was injected unilaterally into the right substantia nigra pars compacta (ML: +2.2 mm AP: -5.3 mm DV: 6.5 mm from bregma and dura of the brain) using a stereotaxic microinjector (BAS BeeHive model MD-1020) under intraperitoneal (IP) Ketamine (90 mg/kg)/Xylazine (10 mg/kg) or inhaled isoflurane anesthesia (induction at 3.7% and maintenance at 2%). Desipramine (20 mg/kg) was injected IP 30 minutes prior to surgery to protect noradrenergic pathways. Sham-operated rats were injected with vehicle under similar conditions. Animals were maintained 2-8 weeks post lesion.
|
Group |
N |
Lesion Type |
Time Maintained |
A |
8 |
6-OHDA |
1 week |
B |
7 |
6-OHDA |
2 weeks |
C |
11 |
6-OHDA |
4 weeks |
D |
7 |
Sham* |
2 weeks |
E |
3 |
Vehicle |
4 weeks |
F |
4 |
None |
--- |
Table 1: Rats used in the experiment grouped according to lesion type (injection into the SN with 6-OHDA or vehicle solution) and length of time rats were maintained post-lesion (1, 2, or 4 weeks). N refers to the sample population, with a total N of 36 rats used in diuresis chamber and cystometry experiments. *Sham-operated animals in Group D include those injected with vehicle as well as those in which we attempted to inject 6-OHDA and did not have a successful lesion.
Measurement of Micturition Patterns using Metabolic Chamber
Animals were placed into metabolic chambers (Tecniplast 3700D005) for a period of 15 hours overnight, and urine was collected into test tubes connected to transducers configured to weigh urine output. The transducers (Grass Force Displacement Transducer FT03C) were connected to a computer where data were measured and stored using Igor Pro 6.34A, UOMA 3.0.2 software. Off-line analysis included calculation of the number of voids, mean time between voids (inter-contraction interval), mean volume per void, and total urine output. Micturition pattern data were collected from each rat every 4-7 days beginning at least one month prior to the SN lesion and continuing until the final cystometry experiment.
Cystometric Investigation of Bladder and EUS Function
Upon completion of the study of micturition patterns in the metabolic chambers, rats were anesthetized with urethane (1.25 g/kg s.c.). A PE 50 tube was inserted into the jugular vein for IV injections. Using a midline abdominal incision, stainless steel electrodes were placed bilaterally and superficially into the EUS muscle to record EUS EMG activity associated with micturition. A PE 90 or FR 3.5 catheter was inserted into the dome of the bladder. This catheter was connected in series to a pressure transducer to record bladder pressure and to a syringe pump (Razel A-99 Syringe Pump or GenieTouch from Kent Scientific Corp.) to infuse physiological saline (0.9% NaCl, 0.04-0.12 ml/min) into the bladder to elicit the micturition reflex. The abdominal incision was closed in layers with sutures and wound clips. Beginning immediately following the surgery, bladder infusion was initiated, and EUS EMG and bladder pressures were digitized (2 kHz) and continuously streamed to disk using Igor Pro 6.34 (Wavemetrics) or LabChart Pro (ADInstruments) for later analysis. Following data collection, the catheter was removed from the bladder and left in place to obtain basement pressure measurements.
Immunohistochemical Analysis of SN Lesion
After completion of cystometric analysis of the bladder and EUS, the animal was perfused under deep anesthesia with urethane with phosphate buffered saline, low pH fixative and high pH fixative (4% paraformaldehyde low pH 6.5, high pH 9 with sodium borate) to fix the nervous tissue. The brain, spinal cord, and bladder were then removed, and immersed in a 30% sucrose solution to cryoprotect before sectioning the tissue. Using a microtome (American Optical Corp. Model 860), brain and spinal tissue was sliced into 40 µm sections and rinsed with 0.1 M phosphate buffer and 1% hydrogen peroxide solution to remove endogenous peroxidase, followed by Tris Buffer with 0.9% NaCl at pH 7.4 to enhance staining intensity. The tissue was then incubated with unlabeled primary antibody (anti-Tyrosine Hydroxylase from rabbit) from Chemicon/Millipore for 3 to 5 days, followed by biotinylated secondary antibody (anti-rabbit IgG) from Vector Laboratories overnight. The Avidin-Biotin peroxidase complex (ABC) reagent was then applied to tissue and incubated for a minimum of 4 hours. The peroxidase was developed with Diammonium Benzadine (DAB) for 20 minutes and reacted with 1% Hydrogen Peroxide to allow for color change and finally, mounted on gelatin slides for view.
Data Analysis
Data were collected via Igor Pro or LabChart Pro and analyzed using customized Igor Pro procedures to measure bladder pressure (BP) and EUS EMG parameters. Each micturition event (ME) was measured individually for the bladder pressure threshold needed for voiding to occur, minimum and peak pressures, inter-contraction interval (ICI), and bladder contraction duration. The ICI was multiplied by the saline infusion rate into the bladder to determine the threshold volume (i.e. the volume of fluid in the bladder prior to each ME). The baseline pressure recorded at the end of each experiment was subtracted from each BP measurement. The EMG parameters were also measured on an individual basis and included the duration of EUS bursting during voiding, the number and frequency of individual burst events, and the area under each curve in order to estimate the level of excitation of the muscle and how quickly it returns to rest. To control for variability in the positioning of electrodes in the EUS and strength of signal received, the area was normalized by dividing by the maximum amplitude at t=0 of a double exponential fit to the sustained EUS activity (rectified and smoothed) following EUS bursting (see Figure 3c).
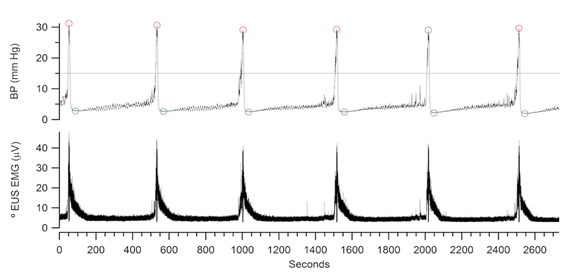
Figure 3a: Example of data collected using Igor software depicting bladder pressure (BP) during a sequence of micturition events (ME) (top) and EUS activity (bottom). These data were analyzed using a custom Igor Pro procedures to measure the start time of each ME, inter-contraction interval in seconds (ICI), minimum and peak bladder pressures, and the area under the EMG waves in relation to baseline activity. The line drawn through the BP record on top indicates the ME detection threshold of the software. The peak and minimum BP of each ME are also circled.
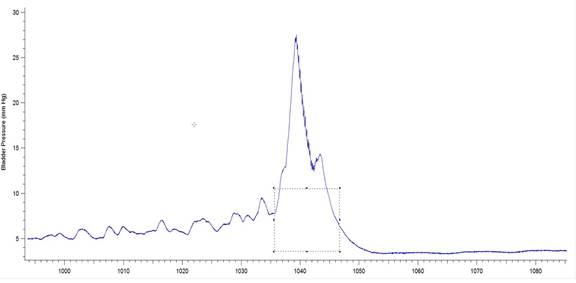
Figure 3b: Example of a bladder pressure record from a 6-OHDA-lesioned animal indicating BP threshold and contraction duration. The box drawn over the pressure wave has been set to measure the threshold pressure (y value indicated by single-headed arrow) as well as the duration of the bladder contraction (indicated by double-headed arrow). The software used was Igor Pro 6.35A, BP_EMG Measure 3.12.
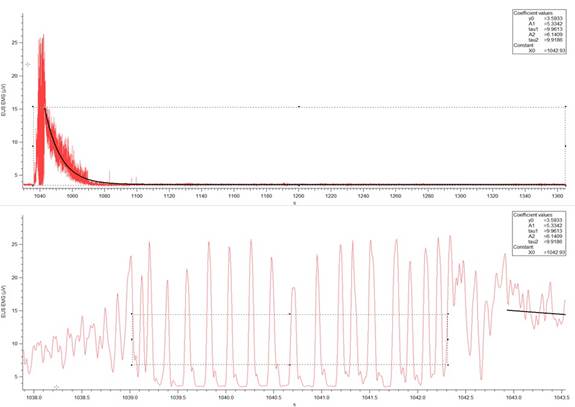
Figure 3c: Example of a EUS EMG record (V over time in seconds) from a 6-OHDA-lesioned animal. (Top) Activity of the muscle during an individual micturition event. The sloped curve represents the calculated exponential decay of the muscle activity. The width of the rectangular box drawn indicates the time from the start of the muscle contraction to the end of relaxation. The bottom of the box is used to measure baseline EMG activity. (Bottom) A horizontal expansion of the section of the EMG activity containing EUS bursting. EUS burst duration is determined by the width of the box and the top of the box indicates the threshold for burst event detection, which was used to determine the EUS burst duration and frequency. The software used was Igor Pro 6.35A, BP_EMG Measure 3.12.
Statistical Analysis
Data values are expressed as mean ± standard error (s.e.). Statistical significance was determined using One-way ANOVA test, followed by Tukey multiple comparison tests for each group of rats used in the diuresis chambers and cystometry studies. Cystometry data were examined for outliers using Chauvenet’s criterion test, and one animal was excluded. Data on rats included in the rotation behavioral analysis were analyzed using the Student paired t-test to compare values before and after administration of 6-OHDA. P values less than 0.05 were considered to be significant.
Results
Histologic Evaluation of 6-OHDA Lesion Site
There was a marked reduction in the level of Tyrosine Hydroxylase (TH) staining on the side of the brain injected with 6-OHDA in the animals observed by Yoga Kammili, indicating loss of dopaminergic neurons. Groups A (1 week 6-OHDA lesion, n=8) and E (4 weeks sham-operated, n=3) were injected on the left side of the brain, Groups B (2 weeks 6-OHDA, n=7) and D (2 weeks sham-operated, n=7) were injected on the right side, and group C (4 weeks 6-OHDA, n=11) was comprised of animals injected on either the left or right side of the brain. No significant difference was observed between the two groups of 4-week lesioned rats. There was no loss of TH immunoreactivity in animals injected with vehicle (data not shown).
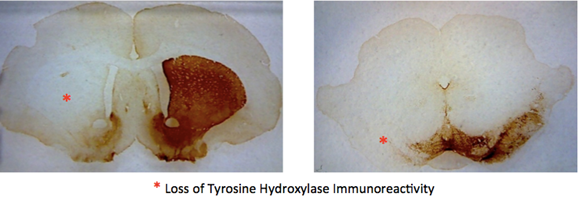
Figure 4: Visualization of the loss of dopamine cells on one side of the rat brain caused by absorption of 6-OHDA.
Assessment of Motor Function
Behavioral analysis was done by Olivia Wang before and after injection of either 6-OHDA or vehicle into the SN. The rats were placed in a large clear container and their movements were recorded over a span of 5 minutes per animal. 4 observers counted the number of left turns made by each rat. Animals before the lesion and sham-operated rats did not exhibit rotation preference (50% left or right turn). After the lesion, however, rats showed a 70-85% preference towards the side of the lesion. The Student paired t-test determined these values to be statistically significant. A different sub-group of sham-operated and lesioned rats were used in this behavioral test.
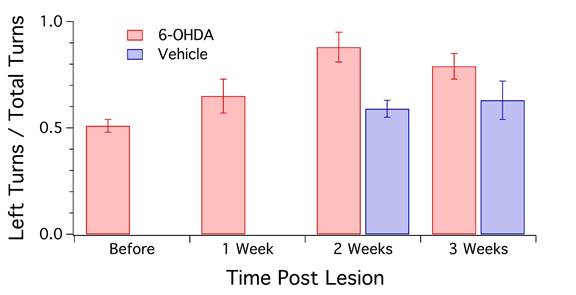
Figure 5: Quantification of the number of left turns observed in animals following injection of 6-OHDA or vehicle on the left side of the brain (n=16). Sham-operated rats (n=3) showed a slight increase in left turns post-surgery. Rats with a 6-OHDA lesion displayed the greatest increase in this behavior at 2 weeks after the injection, and a gradual decline at 3 weeks. However, both 2-week and 3-week data were significantly different from Sham (P<0.05).
Serial Quantification of Urine Output in Diuresis Chambers
6-OHDA lesions produced a significant decrease in ICI in rats at the 2-week survival time but not at the 4-week survival time. At 2 weeks survival, rats treated with 6-OHDA displayed an average ICI of 24.64 ± 1.46 min (mean ± s.e.) compared to 31.34 ± 0.98 min for sham-operated rats (P<0.007). At 4 weeks, average ICI was 29.98 ± 1.79 min. There was also a statistically significant increase in the number of voids in 2-week lesioned animals (33.02 ± 1.66) vs. sham-operated (27.55 ± 1.28) (P<0.05). There was no significant change in void volume or overall urine output between the two groups (P>0.05). In addition, 4-week 6-OHDA-lesioned animals did not show a significant treatment effect in ICI, void volume, or total urine output in relation to sham-operated rats.
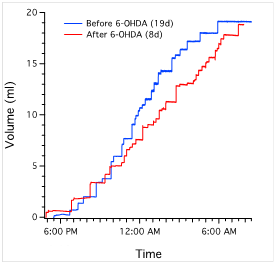
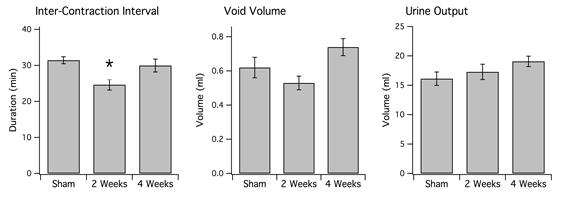
Figure 6b: (Left) Average interval between voids. Single asterisk indicates P<0.007 in comparison to sham-operated rats (n=11). Note that 6-OHDA-treated rats have increased frequency of voiding at 2 weeks (n=7), which disappears at 4 weeks (n=11). There is no significant difference between sham-operated rats and 6-OHDA-treated rats at 4 weeks (P>0.05). (Middle) Average urine volume per void. There was no significant difference in sham-operated rats in comparison to either 2 or 4-week 6-OHDA-treated rats (P>0.05). (Right) Overall urine output averaged across animals of each group. There was also no significant treatment effect in either 6-OHDA-lesioned (n=18) or sham-operated rats (n=10) (P>0.05).
Quantification of Micturition Reflex (Acute Cystometry)
Anesthetized animals with a 6-OHDA lesion also exhibited decreased ICI at 2 weeks but not at 4 weeks. In sham-operated rats (n=10), there was an average ICI of 12.30 ± 0.69 min and volume threshold of 0.49 ± 0.03 ml observed in anesthetized rats. These values were statistically significant in comparison to the ICI (6.51 ± 0.45 min, P<0.05) and volume threshold (0.26 ± 0.02 ml, **P<0.005) for anesthetized 6-OHDA-lesioned rats at 2 weeks (n=6). There was also a significant difference noted between the volume threshold values for 6-OHDA-treated rats at 2 weeks vs. 4 weeks (P<0.05).
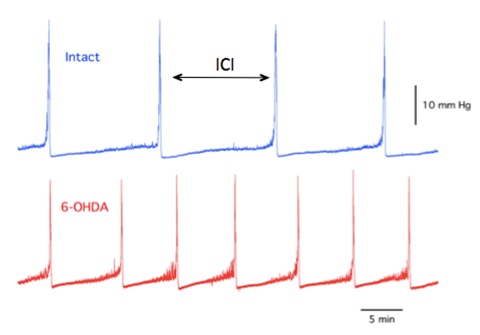
Figure 7a: Example of BP record for naïve (intact) animal (top) and 6-OHDA-lesioned animal (bottom). There is a visible increase in voiding frequency in the latter.
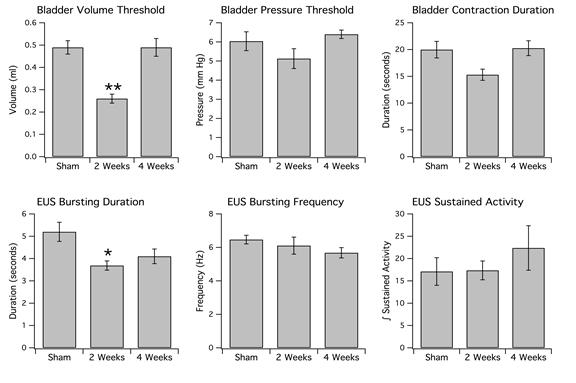
Figure 7b: Top: Bladder parameters. Note that 6-OHDA-lesioned rats at 2 weeks have a significantly decreased threshold volume (left) in comparison to sham-operated rats. The double asterisk indicates a P value <0.005. Although the BP threshold (middle) and contraction duration (right) appear to be reduced at 2 weeks, these values are not statistically significant. Bottom: EUS parameters. Note that the duration of EUS bursting (left) has decreased in 6-OHDA-lesioned rats maintained at 2 weeks vs. sham-operated rats. The single asterisk indicates a P value <0.05. Interestingly, although EUS burst duration is reduced, there is no significant change in frequency of EUS bursting (middle) at 2 weeks in comparison to sham-operated rats (P>0.05). There was also no significant difference in sustained activity (right) amongst the three groups of animals (P>0.05).
Discussion
Results indicate that 6-OHDA lesion of the SN 1) causes a significant increase in voiding frequency in both the awake and anesthetized rat models, an indication of bladder hyperactivity, 2) produces changes in EUS activation characterized by a decrease in duration of EUS bursting during voiding, 3) produces a significant decrease in the volume threshold needed to initiate the micturition reflex, and 4) produces lasting changes in behavior, with no recovery from motor dysfunction over a 4-week time span. These data are consistent with other studies that have confirmed bladder overactivity (Kitta et al, 2006 and Yoshimura et al, 2003) and sustained loss of motor activity (Sakakibara et al, 2012) in PD. However, the present study is the first to quantify EUS function in this model and to show evidence for possible compensatory mechanisms, which serve to restore normal bladder activity following 6-OHDA lesions.
Results from the diuresis chamber experiments provided significant data indicating a decreased ICI at 2 weeks in 6-OHDA-lesioned awake animals. Because these rats voided more frequently at 2 weeks, we would expect there to be a decrease in the volume per void but this was not the case. Though there appears to be a reduced void volume, the decrease was not statistically significant (Figure 6b) and was much less than the decrease in void volume in 6-OHDA-lesioned rats observed by Yoshimura et al (2003). However, it is important to note that our experimental setup was slightly different. For example, rats in the present study were placed in diuresis chambers for 15 hours during their light (awake) cycle whereas Yoshimura et al placed rats in the chambers for 24 hours, encompassing both their light and dark cycles, which could influence their void volume. Awake animals are not voiding purely reflexively, but little is understood about the mechanisms underlying the difference in micturition between awake and anesthetized rats.
Cystometry experiments revealed a 50 percent reduction in bladder volume threshold at 2 weeks in 6-OHDA-lesioned rats, indicating that less fluid was necessary to initiate the micturition reflex. Surprisingly, this was not reflected in the bladder pressure threshold, which was not significantly reduced. Also, there was no significant reduction in bladder contraction duration, although it might be expected that this value would be reduced given that the EUS burst duration is decreased in 6-OHDA-treated animals at 2 weeks. A shorter EUS burst duration suggests that the urethra is opened for a shorter period of time. This would be consistent with decreased void volume and urine output as well, which is not seen. Perhaps a larger sample size would produce results indicating that there is in fact a significant change in BP threshold and void volume. However, it may also be possible that there is residual volume in the bladder following each void, indicated by the marked decrease in volume threshold and ICI. If this hypothesis were correct, then less fluid entering the bladder would be sufficient to reach a similar pressure threshold. In addition, other studied have also reported that there was no significant difference in BP threshold in 6-OHDA-treated vs. sham-operated rats (Kitta et al, 2012).
The results presented in Figure 7b for BP threshold and contraction duration are also impacted by the difficulty in measuring these values in a BP record, particularly in animals that exhibited frequent spontaneous non-voiding bladder contractions prior to voiding. This was a common occurrence observed in the present study in animals with a 6-OHDA lesion (see Figure 8).
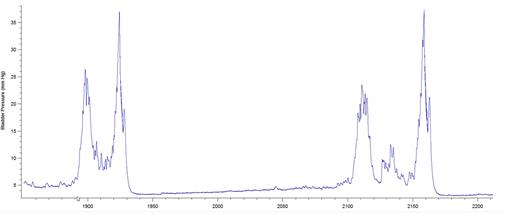
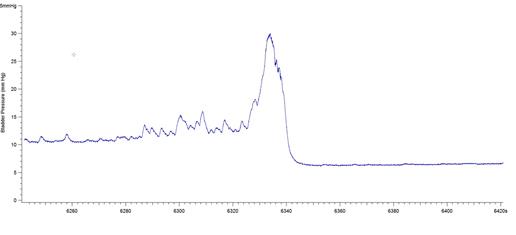
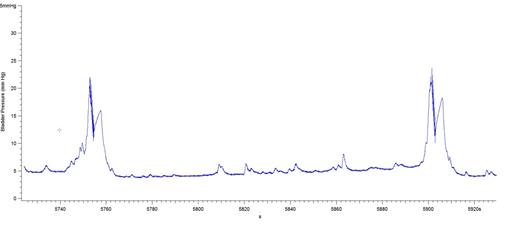
Figure 8: BP records from 3 different 6-OHDA-lesioned animals exhibiting spontaneous smooth muscle contractions (non-voiding) prior to micturition. Note that it is difficult to estimate precisely when the ME begins and ends, leading to imprecise calculations of bladder pressure threshold and contraction duration. The software used was Igor Pro 6.35A.
In regards to EUS function, it is important to note the current hypothesis that spinal reflexes play a role in the coordination of EUS behavior. There appears to be input coming from L6-S1 segments of the spinal cord which control tonic activity of the EUS, while EUS bursting activity may be controlled by a pattern generator located in L3-4 (Chang et al, 2007). The presence of a EUS spinal controlling center, as supported by observations in rats with spinal cord injury (Abud et al, 2015), would indicate further involvement of other systems in the micturition reflex besides those already mentioned (i.e. DA D1/D2 receptor activity and hypothalamic DA input). Our observation of reduced EUS burst duration at 2 weeks in 6-OHDA-treated rats would be consistent with the hypothesis of input from L3-4 that influence EUS bursting independent of other aspects of EUS activity. While it appears that the urethra plays only a minor role in the micturition reflex, the EUS is still a subject for further study due to its ability to reorganize in the presence of spinal cord injury. For example, following spinal transection and interruption of input from the PAG and PMC, the micturition reflex was completely absent but reemerges following some mechanism of synaptic reorganization (Chang et al, 2007). This compensation allows EUS bursting activity to return, which is linked to bladder factors such as the duration of bladder contraction and void volume. Therefore, it is possible that the compensatory mechanisms observed in rats in this study leading to return of function at 4 weeks may influence activity at the level of the spinal cord.
Another important factor that must be considered when analyzing bladder and EUS function is the effect of serotonergic pathways on micturition. At the start of the experiments, Desipramine was used in order to block the uptake of 6-OHDA at noradrenergic receptors. However, Desipramine is of the tricyclic antidepressant (TCA) class of molecules, which also exert an effect on serotonergic (5-HT) reuptake as well (Zhou et al, 2007). Serotonin is known to modulate micturition in anesthetized rats, producing increased volume threshold, prolonged EUS bursting, lowered BP threshold, and increased sustained activity (Cheng & de Groat, 2010). However, it is unlikely that the results of this study reflect such an influence because we have not observed a decrease in BP threshold, or an increase in EUS burst duration and sustained activity in 6-OHDA-lesioned animals at 2 weeks. While we may rule out modulation by serotonin as an influencing factor in the results, it is still challenging to account for the lack of change in void volume in this group of animals. Yoshimura et al (2003) observed significantly smaller bladder capacity in 6-OHDA-lesioned rats, which was consistent with the lowered void volume recorded in the same animals. Although the bladders of rats used in experiments of the present study were weighed following perfusion with fixative (results not shown), there was no exact quantification of bladder capacity or void volume during cystometry. Therefore, there is a possibility that with additional parameters, there may be sufficient data to verify treatment-dependent changes in void volume and bladder contraction duration.
These results indicate not only that the 6-OHDA-lesioned rat is a good model for PD, but also that there is a possibility for recovery of LUT function in patients affected by detrusor overactivity, nocturia, and other symptoms of bladder hyperactivity. Compensatory mechanisms may be occurring at the level of the PAG and PMC, which are then carried out by the spinal micturition control centers hypothesized by Chang et al (2007). Following loss of DA, there may be reorganization of the micturition circuitry in the midbrain after 2 weeks, which allow GABAergic inhibition of micturition to reemerge. This would allow the ICI and volume threshold of 6-OHDA-lesioned rats to return to normal values.
Conclusion
This study confirms that bladder hyperactivity is induced following 6-OHDA lesion of the substantia nigra pars compacta. Symptoms of LUT dysfunction manifested as increased frequency of micturition (decreased ICI) with decreased duration of voiding (decreased duration of EUS bursting), consistent with the pathophysiology of PD. These lesion-induced changes were observed at 2 weeks post-lesion but not at 4-weeks, indicating a transient effect of the lesion in rats. Thus, future studies should aim to recreate these results and determine the possible mechanisms underlying the transient LUT dysfunction observed at 2 weeks and not at 4 weeks, as well as those leading to improved function of the bladder at 4 weeks. Understanding of both these systems might aid in the generation of further treatment options for bladder hyperactivity in PD.
References
Abud, E. M., Ichiyama, R. M., Havton, L. A., & Chang, H. H. Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. Am J Physiol Renal Physiol, 308.9 (2015), F1032-1040.
Araki, I. and Kuno, S. Assessment of Voiding Dysfunction in Parkinson's Disease by the International Prostate Symptom Score. Journal of Neurology, Neurosurgery & Psychiatry, 68.4 (1999): 429-33.
Chang, H. Y., Cheng, C. L., Chen, J. J., & de Groat, W. C. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol, 292.3 ((2007), F1044-1053.
Cheng, C. L., & de Groat, W. C. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. Am J Physiol Renal Physiol, 298.3 (2010). F771-778.
De Groat, W. and Wickens, C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol (Oxf.), 207.1 (2013): 66-84.
Kitta, T., Chancellor, M., de Groat, W., Kuno, S., Nonomura, K. and Yoshimura, N. Suppression of Bladder Overactivity by Adenosine A2A Receptor Antagonist in a Rat Model of Parkinson Disease. The Journal of Urology, 187 (2012): 1890-1897.
Kitta, T., Kakizaki, H., Furuno, T., Moriya, K., Tanaka, H., Shiga, T., Tamaki, N., Yabe, I., Sasaki, H., and Nonomura, K. Brain Activation During Detrusor Overactivity in Patients With Parkinson’s Disease: A Positron Emission Tomography Study. The Journal of Urology, 175 (2006): 994-998.
Kitta, T., Mastumoto, M., Tanaka, H., Mitsui, T., Yoshioka, and M. Nonomura, K. GABAergic mechanism mediated via D1 receptors in the rat periaqueductal gray participates in the micturition reflex: an in vivo microdialysis study. European Journal of Neuroscience, 27 (2008): 3216-3225.
Moore, R. and Bloom, F. Central Catecholamine Neuron Systems: Anatomy and Physiology of the Dopamine Systems. Ann. Rev. Neurosci. 1 (1978): 129-169.
Fowler, C., Griffiths, D., and de Groat, W. The neural control of micturition. Nature Reviews: Neuroscience, 9 (2008): 453-466.
Ogawa, T., Seki, S., Masuda, H., Igawa, Y., Nishizawa, O., Kuno, S., Chancellor, M., de Groat, W., and Yoshimira, N. Dopaminergic Mechanisms Controlling Urethral Function in Rats. Neurourology and Urodynamics, 25 (2006): 480-489.
Sakakibara, R., Tateno, F., Kishi, M., Tsuyuzaki, Y., Uchiyama, T., and Yamamoto, T. Pathophysiology of bladder dysfunction in Parkinson’s disease. Neurobiology of Disease, 46 (2012): 565-571.
Skagerberg, G. and Lindvall, O. Organization of Diencephalic Dopamine Neurones Projecting to the Spinal Cord in the Rat. Brain Research, 342 (1985): 340-351.
Takada, M., Li, Z.K., and Hattori, T. Single thalamic dopaminergic neurons project to the neocortex and spinal cord. Brain Research, 455 (1988): 346-352.
Yoshimura, N., Sasa, M., Yoshida, and O., Takaori, S. Dopamine D-1 Receptor-Mediated Inhibition of Micturition Reflex by Central Dopamine from the Substantia Nigra. Neurourology and Urodynamics, 11 (1992): 535-545.
Yoshimura, N., Kuno, S., Chancellor, M., de Groat, W., and Seki, S. Dopamineric mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. British Journal of Pharmacology, 139 (2003): 1425-1432.
Zhou, Z., Zhen, J., Karpowich, N., Goetz, R., Law, C., Reith, M., and Wang, D. LeuT-Desipramine Structure Reveals How Antidepressants Block Neurotransmitter Reuptake. Science, 317.5843 (2007): 1390-1393.

