The Effect of Microbial Contamination upon the Plasticity of Ceramic Clays
John Henrikson, Ted Fleming*
Bradley University
Keywords: clays, microbial growth, plasticity, shear strength
Abstract
Relatively little evaluation has been conducted on the effects of individual microbes present in ceramic clays. In this study, bacteria present in dry ingredients and also aged clay were surveyed and identified as to the genus level using a combination of culture and microscopic techniques. Isolates of predominant bacteria were individually evaluated by reintroduction into sterile dry ingredients and aging of the wet clays. Subsequently clay samples were subjectively assessed for wet pliability and quantitatively tested for wet plasticity and shear strength after firing. Following a 10-day aging period, experimental clays were found to be 10 percent more plastic than control clays (sterile) and demonstrated 1.5 -13.2 percent lower cured shear strength.
Introduction
There is anecdotal evidence that the aging of clay improves its plasticity: most artisans who work with the material constantly notice changes in the workability or physiochemical properties. In addition, previous studies have ascertained the improvement in plasticity due to microbial treatment of clay (Naga et. al., 1998). There are many methods used to age clays, ranging from ancient storage in underground pits to modern techniques involving storage in Igloo® coolers; all methods provide time for clays to somehow become more workable. Different theories among ceramic artists related to the aging process include changes to the clay ions, changes in moisture and temperature, or microbial products that may help the clay particles bind more closely together. Previous studies evaluated mixed or indigenous populations of microbes in native clays having varying physiochemical properties (Gaidzinski et al., 2009). This study evaluated the effect of growth of individual microbes under controlled conditions upon specific properties of aged clays. It was hypothesized that specific microbes are predominantly responsible for increased clay plasticity and that there is a positive correlation between the presence of those microbes in aged clay and the working plasticity of that clay. Aged clays were evaluated for plasticity and fired shear strength relative to sterile controls.
Method
Sampling and characterization of isolates
Dry clay ingredients were cultured to determine background contamination. Aged clay that was anecdotally known to be of superior quality was the source of microbial cultures used in this study. An initial survey of background microbes present in dry clay ingredients was performed by diluting water that was kept above aged clay in a storage tank and culturing (30oC, 48 hrs) on Tryptic Soy agar (TSA), phenyl ethyl alcohol agar (PEA), and MacConkey agar (MAC). Selective growth on PEA and MAC yielded preliminary information related to microbial identities. Numerous colonies were present, and total counts of gram positive and gram negative bacteria (cfus/ml) were determined and evaluated for relative frequency of occurrence. The four most frequently-occurring isolates, 1-7, 1-11, 2-3, and 2-6, were identified as to genus level using standard biochemical tests and were grown overnight in nutrient broth (37o C with aeration) prior to inoculation into sterile ingredients. It was presumed that the most frequently-occurring isolates would have the greatest effect upon clay ingredient aging.
Inoculation of sterile clays and evaluation of aged clays
Cells were concentrated by centrifugation (15 min., 10,000 rpm), re-suspended in sterile water-soy milk, and used to inoculate uniform batches of sterile dry ingredients (20g dried fire clay, 20g dried gold art clay, 10ml deionized water, 20 ul soy milk). The experimental dilution of the soy milk mimicked the dilution customarily used by the artisan (8 oz. bottle of soy milk per batch of clay). Control clay had no added cells. All clays were placed in sterile jars and incubated (aged) at 30o C for 10 days. Aged clays were subjectively assessed for pliability by simple coiling of uniform 76mm x 6 mm extrusions. Wet plasticity of replicate clay extrusions was measured relative to the control clay (device fabricated for non-destructive analysis). These same clay extrusions were air dried and subsequently fired using normal ceramic production techniques, and shear was measured relative to the control clay (device fabricated for destructive analysis). Cross sections of sheared extrusions were examined microscopically.
Results
No microbial growth occurred after plating samples from separate dry ingredients, indicating no culturable background contamination. Samples from aged clays showed significant bacterial contamination. Forty-six bacterial isolates were obtained from the clay water. Among the bacterial isolates, four bacteria were found to predominate (20 - 100% frequency of occurrence on selective media, data not shown). All were gram negative bacilli and were identified as members of genus Pseudomonas. No fungi were cultured. All isolates produced colonies having some degree of mucoid appearance.
Sample
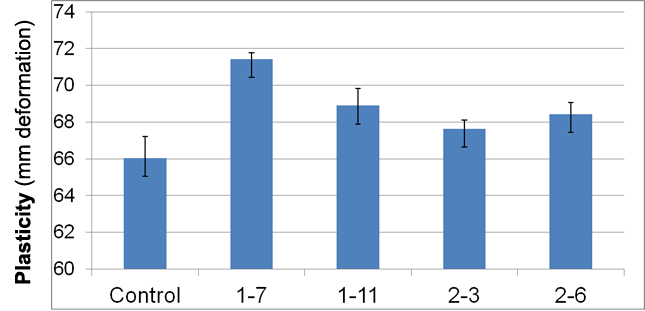
Figure 1. Wet plasticity of incubated clays (aged 30o C, 10 days).
Sample
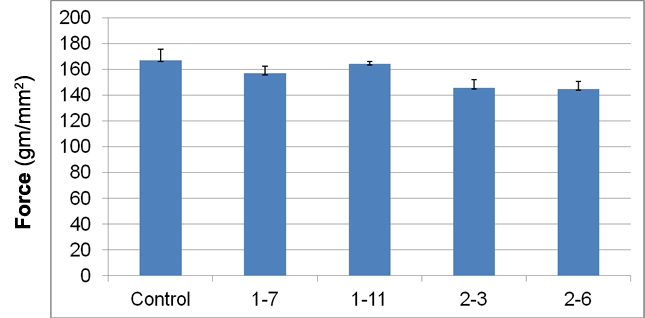
Figure 2. Shear strength of fired clay extrusions.
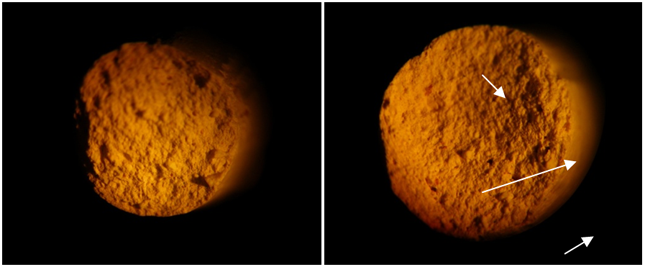
Figure 3. Cross sections of sheared clay extrusions Control (left), experimental (right), 25x magnification.
Discussion
Data suggest that bacterial contamination of clays occurs during and after hand mixing of dry ingredients with water, as cultures prepared from undiluted dry ingredients exhibited no microbial growth. .
All samples of clays aged with one of the four bacteria were found to be more pliable (subjective evaluation) and had 2.4 – 8.2 percent greater wet plasticity measured relative to the control (Figure 1). It was noted that colonies of isolate number 1-7, which yielded the greatest plasticity (+8.2%), exhibited a very mucoid appearance. Isolates other than 1-7 demonstrated wet plasticity that was not significantly different than that of the control clay. Data appear to support the hypothesis that specific microbes may be associated with increased clay plasticity. A negative correlation was observed between greater concentrations of those microbes in aged clays and the working plasticity of the clays after clays were inoculated with high concentrations (> 109 cells/ ml) of bacteria (data not shown). This suggests that microbial effects upon clays may not be concentration-dependent.
Fired extrusions were measured to determine if microbial growth affected the important property of shear strength. All experimental fired clays exhibited somewhat lower cured shear strength (1.5 – 13.2%) relative to the control samples; however the difference was not significant (Figure 2). Microscopic examination of cross-sections of sheared extrusions demonstrated a greater number of void spaces in experimental clays relative to control (Figure 3), however it was not possible to quantify this finding. Void spaces in the fired clays may be the result of combustion of bacterial cells, cellular secretions, and metabolites during clay firing and would be expected to contribute to the observed lower shear strength.
Previous studies have suggested that some microorganisms found in clays secrete polysaccharides, which may bridge and aggregate the clay particles and promote increased plasticity (Abajo, 2000). Clay improvement by these bacteria was attributed mainly to mucilaginous exopolysaccharides produced during growth (Groudeva & Groudeva, 1995). Polysaccharides secreted by mixed populations of bacteria (Uz et al., 2010) or isolates of Bacillus mucilaginosus (Kakoshoko et al., 2005) have been associated with improved plasticity of clay samples. Although the bacteria isolated during this study exhibited a mucoid appearance on TSA plates, polysaccharide assays performed (technique modified from Xiao et al., 2006) upon the liquid of bacterial broth cultures and upon plated colonies themselves failed to indicate polysaccharide down to 0.0625 percent (the limit of detection; data not shown). In addition, Bacillus mucilaginosus was not among the bacteria isolated and used for clay aging. Therefore it was not possible to conclude during this study that bacterial secretions significantly acted to lubricate and aggregate particles in experimental clays. A similar lack of correlation between the presence of microorganisms and changed clay properties was previously noted (Gaidzinski et al., 2009).
Future efforts could involve investigation of the aging affects of other microbes, extended aging times, and detection of a range of microbial products in plastic clays.
References
Abajo, M.F., 2000. Manual sobre fabricacion de baldosas, tejas y ladrillos. Beralmar, Columbia, 360 pp.
Kakoshko, E., Dyatlova, E., Biryuk, V., Zayats, N., 2005. Effect of microbiological treatment on technological properties of clays with different Mineralogical Compositions. Glass and Ceramics, 62 (5): 162-166.
Uz, V., Ozdag, H., Ihan, S., Ceylan, A. Isik, I., 2010. The effects of microorganisms on the plasticity and strength of clays. Journal of Ceramic Processing Research, 11 (5): 606-611.
Xiao, Z., Storms, R., & Tsang, A., 2006. A quantitative starch? Iodine method for measuring alpha-amylase and glucoamylase activities. Analytical biochemistry, 351(1), 146-148.
Gaidzinski, R., Osterreicher-Cunha, P., Duailibi Fh, J., & Tavares, L. M., 2009. Modification of clay properties by aging: Role of indigenous microbiota and implications for ceramic processing. Applied Clay Science, 43(1), 98-102.
Groudeva, V. I., & Groudev, S. N., 1995. Microorganisms improve kaolin properties. American Ceramic Society Bulletin, 74(6), 85-89.
Naga, S.M., El-Masry, H.G., Mansour, F.A., El-Sayed Abdel-Aziz, M., 1998. Use of microorganisms for improvement of Egyptian kaolins ceramic properties. Industrial Ceramics, 18, 159-166.
|